Procedure for Submission of Research protocols
Principal Investigator
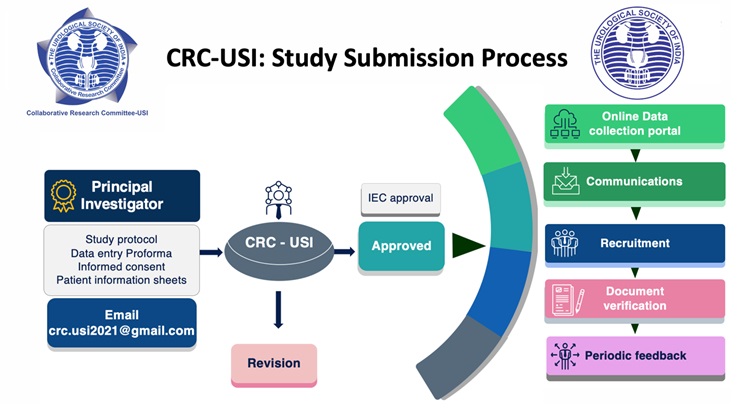
If a member (i.e., Principal investigator (PI)) decides to perform a multi-center collaborative study. The PI can approach or communicate the committee and submit the study protocol to the following e-mail: crc.usi2021@gmail.com
There is no specific time frame for submission of such proposals and all USI members interested in collaborative research are encouraged to send in their proposals in brief as and when they are ready with study protocol (Title, Introduction, Aims and Objectives, Proposed methodology, Data collection Performa). Informed consent and patient information sheets (including vernacular language) shall also be needed once project is approved.
The study will be presented to the committee for approval. If there are revisions, the committee will suggest accordingly. Once the study protocol is approved, the PI needs to get IEC approval. The study approval will be communicated to USI. USI IT team will coordinate with the PI and provide a central database platform for data entry and storage pertaining to the study. Meanwhile, PI will communicate and inform other members for enrollment into the study through USI. The study protocol will be published in the USI newsletter and communicated officially to all the members through e-mail. PI can personally communicate and take help from committee if necessary. Members interested in the study will contact the PI of the study for approval. PI periodically needs to inform the committee regarding the members or institutes enrolled and the timely progress of the study.
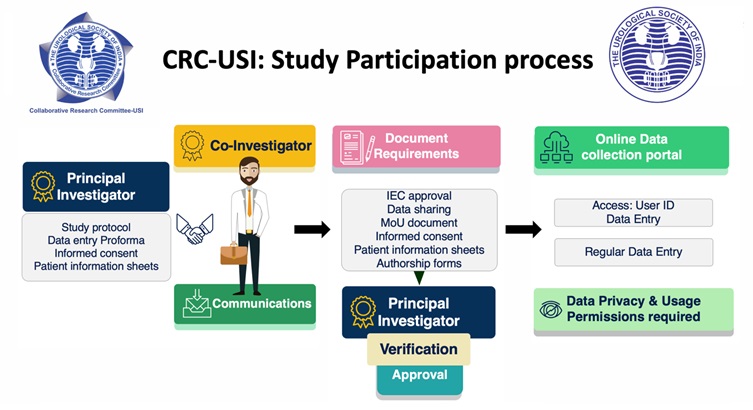
Co-Investigator
Members (i.e., Co-investigators) who are keen to enrol in the study need to communicate with the PI. PI shall provide all the necessary documents required for ethical approval at each individual participating center of the study. The co-investigators need to get IEC approval, sign a Letter of Understanding or authorship related documents and the terms and conditions pertaining to the study before enrolment. Once the verification is confirmed by the PI, a secure access for online data entry will be provided. Regular entry of data is required. The Co-investigator will have access to his or their institute data only.